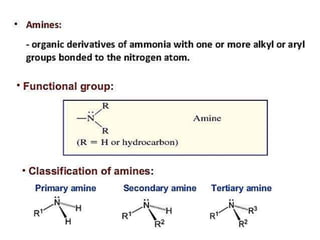
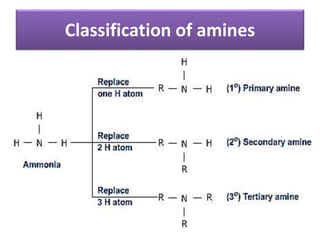
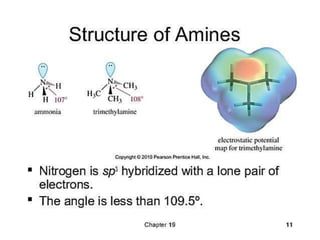
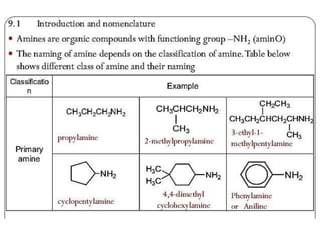
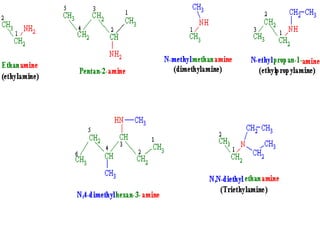
Compound containing nitrogen
- 1. Compound containing Nitrogen By Azeem
- 2. Nitro Compound General method of preparation- 1. Nitration of alkanes 2. From alkyl halides 3. From salts of α-halogeno carboxylic acid 4. From primary amines 5. From a-nitroalkenes(2methyl nitropropene) 6. From oximes(aldoxime +O in trifluoroperoxy acetic acid)
- 3. Reaction of nitroalkane 1. Reduction 2. Hydrolysis 3. Halogenation 4. Reaction with nitrous acid 5. Condensation with aldehydes and ketones Nitro Compound
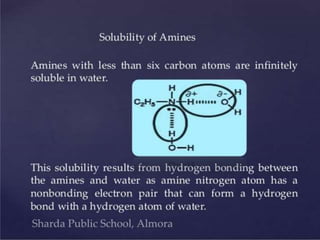
- 7. Physical Properties of Amines • The methylamines (mono-, di-, tri-) and ethylamine are gases at room temperature , other amines are liquid at room temperature. • Amines are irritating to the skin, eyes, and mucous membranes and are toxic by ingestion. • Aromatic amines are generally toxic. Amines are readily absorbed through the skin and affect both the blood and the nervous system. • Amines B.P are higher than alkane but not in alcohol.
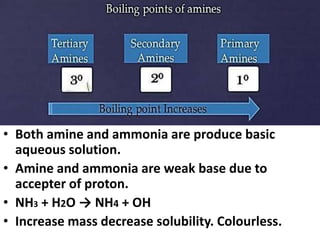
- 8. • Both amine and ammonia are produce basic aqueous solution. • Amine and ammonia are weak base due to accepter of proton. • NH3 + H2O → NH4 + OH • Increase mass decrease solubility. Colourless. Physical Properties of Amines
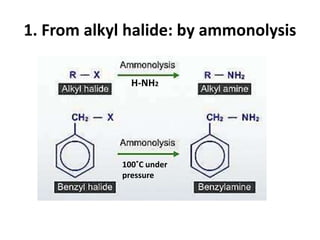
- 13. Method of preparation of amines 1. From alkyl halide: by ammonolysis 2. From alkyl halide: Gabriel phthalimide synthesis(alkylation of phthalimide) 3. From oximes, alkyl cyanides, amides and nitro compounds: by reduction (a) From oximes (b) From alkyl cyanide(alkyl nitriles) (c) From amides (d) From nitro compounds (e) From amides: by hoffman bromamide degradation
- 14. 1. From alkyl halide: by ammonolysis H-NH2 100˚C under pressure
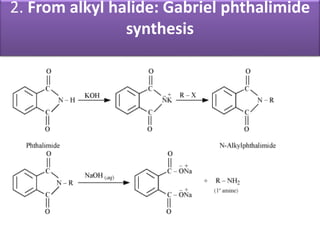
- 15. 2. From alkyl halide: Gabriel phthalimide synthesis
- 16. 3. From oximes, alkyl cyanides, amides and nitro compounds: by reduction
- 17. a. From oximes • Aldoxime or ketoximes are reduce by sodium and ethanol to primary amine. • R- CH= N-OH + 4[H] → R-CH2-NH2 + H2O • Ketoxime. • Acetaloxime.
- 18. b. From alkyl cyanide(alkyl nitriles) • Alkyl cyanide on reduction by Na and ethanol give primary amine. • R-C ≡ N + 4[H] → R-CH2-NH2 • Eg. Acetonitrile and Propanenitrile.
- 20. d. From nitro compound • Nitro compounds are reduced by tin(Sn) or iron and con HCl to corresponding primary amines. • R- NO2 + 6[H] → R- NH2 + 2H2O • Eg. Nitrobenzene. • Nitropropane.
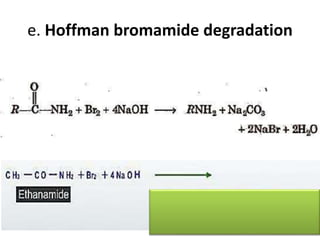
- 21. e. Hoffman bromamide degradation
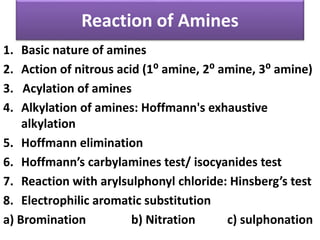
- 22. Reaction of Amines 1. Basic nature of amines 2. Action of nitrous acid (1⁰ amine, 2⁰ amine, 3⁰ amine) 3. Acylation of amines 4. Alkylation of amines: Hoffmann's exhaustive alkylation 5. Hoffmann elimination 6. Hoffmann’s carbylamines test/ isocyanides test 7. Reaction with arylsulphonyl chloride: Hinsberg’s test 8. Electrophilic aromatic substitution a) Bromination b) Nitration c) sulphonation
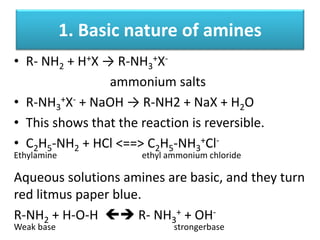
- 23. 1. Basic nature of amines • R- NH2 + H+X → R-NH3 +X- ammonium salts • R-NH3 +X- + NaOH → R-NH2 + NaX + H2O • This shows that the reaction is reversible. • C2H5-NH2 + HCl <==> C2H5-NH3 +Cl- Ethylamine ethyl ammonium chloride Aqueous solutions amines are basic, and they turn red litmus paper blue. R-NH2 + H-O-H R- NH3 + + OH- Weak base strongerbase
- 24. 2. Action of nitrous acid • Primary, secondary & tertiary amines react differently with nitrous acid. • Since nitrous acid is unstable, it is prepared in situ(Latin- reaction mixture) at 273K- 278K. • NaNO2 + HCl → NaCl + HNO2 • Except methyl and ethylamine, all primary amine give aliphatic diazonium salt.
- 25. • Primary amine: (HNO2) eg. Aniline R – NH2 + NaNO2 + 2HCl → [ R- N2 +Cl-] + NaCl + 2H2O Alkyl diazonium chloride • Secondary amine: (HNO2)eg. diethylamine R2 – NH + NaNO2 + HCl → R2N-N=O + NaCl + 2H2O N- nitroso dialkylamine • Tertiary amine: (HNO2)eg. triethylamine R3 – N + NaNO2 + HCl → [ R3- NH]+NO2 - + NaCl trialkyl ammonium nitrate 2. Action of nitrous acid
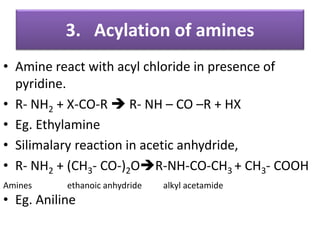
- 26. 3. Acylation of amines • Amine react with acyl chloride in presence of pyridine. • R- NH2 + X-CO-R R- NH – CO –R + HX • Eg. Ethylamine • Silimalary reaction in acetic anhydride, • R- NH2 + (CH3- CO-)2OR-NH-CO-CH3 + CH3- COOH Amines ethanoic anhydride alkyl acetamide • Eg. Aniline
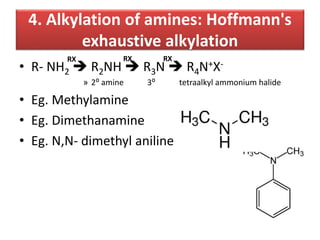
- 27. 4. Alkylation of amines: Hoffmann's exhaustive alkylation • R- NH2 R2NH R3N R4N+X- » 2⁰ amine 3⁰ tetraalkyl ammonium halide • Eg. Methylamine • Eg. Dimethanamine • Eg. N,N- dimethyl aniline RXRX RX
- 28. 5. Hoffmann elimination • Tetra alkyl ammonium halide on heating with moist Ag2O form tetra-alkyl ammonium hydroxides. • This is deliquescent crystalline solid and basic in nature. (of a solid) tending to absorb moisture from the air and dissolve in it. • On heating undergo β-elimination to form alkene, tertiary amine and water. • C2H5N-(CH3)3 +I- + AgOH C2H5-N+(CH3)3OH- + AgI Ethyltrimethyl ammonium iodide • C2H5-N+(CH3)3OH- CH2=CH2 + (CH3)3N + H2O Ethyltrimethyl ammonium hydroxide
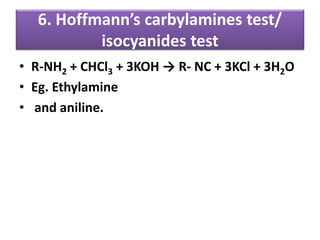
- 29. 6. Hoffmann’s carbylamines test/ isocyanides test • R-NH2 + CHCl3 + 3KOH → R- NC + 3KCl + 3H2O • Eg. Ethylamine • and aniline.
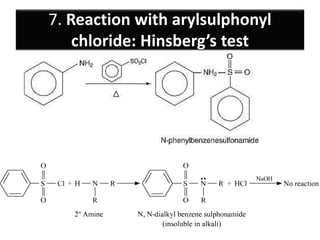
- 30. 7. Reaction with arylsulphonyl chloride: Hinsberg’s test
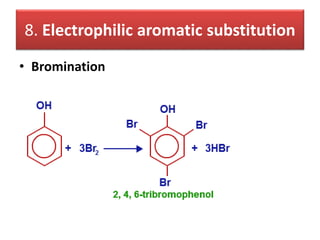
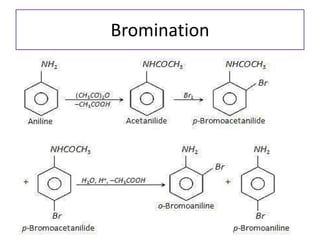
- 31. 8. Electrophilic aromatic substitution • Bromination
- 32. Bromination
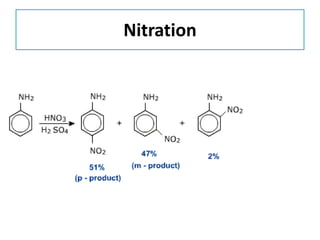
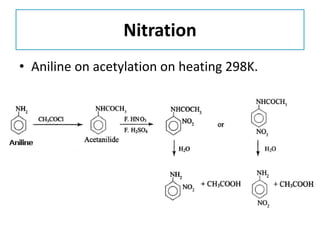
- 33. Nitration
- 34. Nitration • Aniline on acetylation on heating 298K.
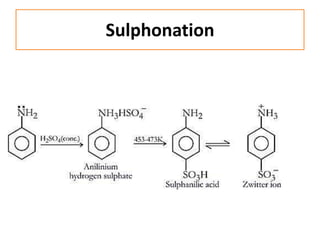
- 35. Sulphonation
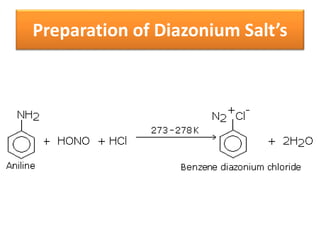
- 36. Preparation of Diazonium Salt’s
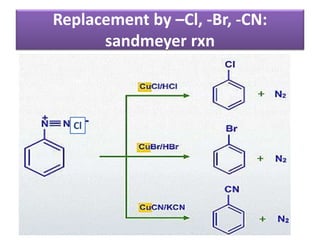
- 37. Reaction of Diazonium salts A. Reaction involving replacement of diazonium group 1. Replacement by –Cl, -Br, -CN: sandmeyer rxn 2. Gettermann reaction: replacement by –Cl, Br: 3. Balz- Schiemann: Replacement by –F.–I,-H, - OH, -NO2 B. Reactions involving retention of diazonium Group: Coupling reaction
- 38. Replacement by –Cl, -Br, -CN: sandmeyer rxn Cl
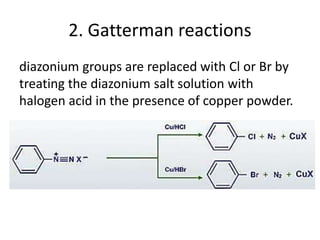
- 39. 2. Gatterman reactions diazonium groups are replaced with Cl or Br by treating the diazonium salt solution with halogen acid in the presence of copper powder.
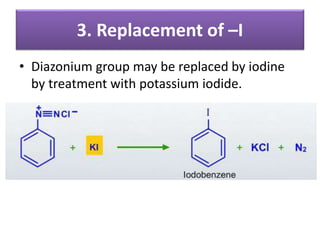
- 40. 3. Replacement of –I • Diazonium group may be replaced by iodine by treatment with potassium iodide.
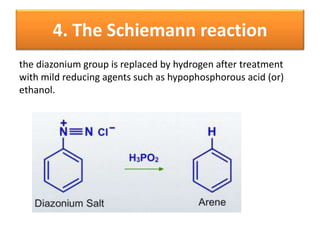
- 41. 4. The Schiemann reaction the diazonium group is replaced by hydrogen after treatment with mild reducing agents such as hypophosphorous acid (or) ethanol.
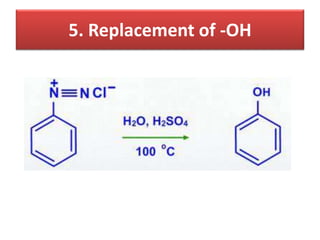
- 42. 5. Replacement of -OH
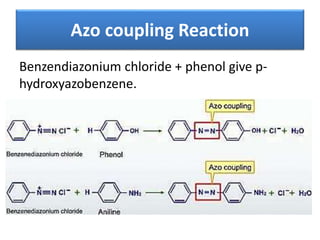
- 43. Azo coupling Reaction Benzendiazonium chloride + phenol give p- hydroxyazobenzene.
- 44. Importance of Diazonium salts • Arene diazonium salts are intermediates to introduce –F, -Cl, -Br, -I, -CN, -H, -OH, NO2 groups in aromatic ring. • Azo comounds are strongly coloured(red, yellow, orange, blue) and used as dyes.








![a. From oximes
• Aldoxime or ketoximes are reduce by
sodium and ethanol to primary amine.
• R- CH= N-OH + 4[H] → R-CH2-NH2 + H2O
• Ketoxime.
• Acetaloxime.](https://image.slidesharecdn.com/70nnekcprzkmqrjvxmjk-signature-016162306cab773303228479b063befdf29baf20ea52f9a91b002e88ad5a07bd-poli-151129105602-lva1-app6891/85/Compound-containing-nitrogen-17-320.jpg)
![b. From alkyl cyanide(alkyl nitriles)
• Alkyl cyanide on reduction by Na and
ethanol give primary amine.
• R-C ≡ N + 4[H] → R-CH2-NH2
• Eg. Acetonitrile and Propanenitrile.](https://image.slidesharecdn.com/70nnekcprzkmqrjvxmjk-signature-016162306cab773303228479b063befdf29baf20ea52f9a91b002e88ad5a07bd-poli-151129105602-lva1-app6891/85/Compound-containing-nitrogen-18-320.jpg)
![c. From amides
4[H]](https://image.slidesharecdn.com/70nnekcprzkmqrjvxmjk-signature-016162306cab773303228479b063befdf29baf20ea52f9a91b002e88ad5a07bd-poli-151129105602-lva1-app6891/85/Compound-containing-nitrogen-19-320.jpg)
![d. From nitro compound
• Nitro compounds are reduced by
tin(Sn) or iron and con HCl to
corresponding primary amines.
• R- NO2 + 6[H] → R- NH2 + 2H2O
• Eg. Nitrobenzene.
• Nitropropane.](https://image.slidesharecdn.com/70nnekcprzkmqrjvxmjk-signature-016162306cab773303228479b063befdf29baf20ea52f9a91b002e88ad5a07bd-poli-151129105602-lva1-app6891/85/Compound-containing-nitrogen-20-320.jpg)

![• Primary amine: (HNO2) eg. Aniline
R – NH2 + NaNO2 + 2HCl → [ R- N2
+Cl-] + NaCl + 2H2O
Alkyl diazonium chloride
• Secondary amine: (HNO2)eg. diethylamine
R2 – NH + NaNO2 + HCl → R2N-N=O + NaCl + 2H2O
N- nitroso dialkylamine
• Tertiary amine: (HNO2)eg. triethylamine
R3 – N + NaNO2 + HCl → [ R3- NH]+NO2
- + NaCl
trialkyl ammonium nitrate
2. Action of nitrous acid](https://image.slidesharecdn.com/70nnekcprzkmqrjvxmjk-signature-016162306cab773303228479b063befdf29baf20ea52f9a91b002e88ad5a07bd-poli-151129105602-lva1-app6891/85/Compound-containing-nitrogen-25-320.jpg)