10003553
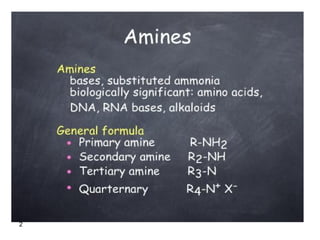
- 1. Amines 1
- 2. 2
- 3. 3
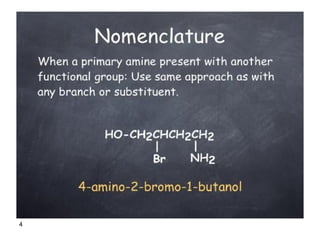
- 4. 4
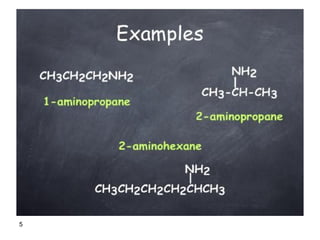
- 5. 5
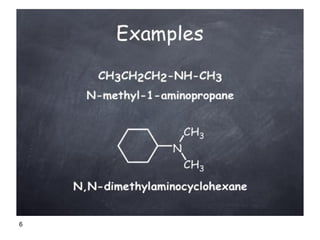
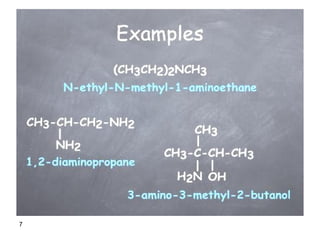
- 6. 6
- 7. 7
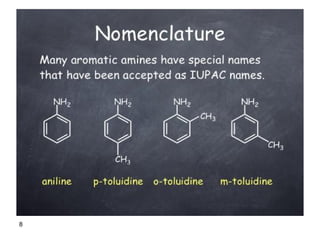
- 8. 8
- 9. 9
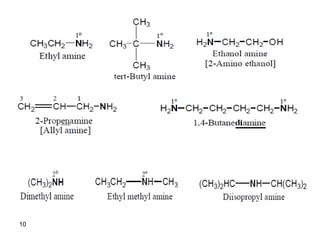
- 10. 10
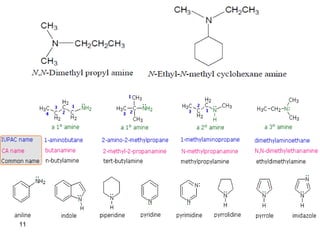
- 11. 11
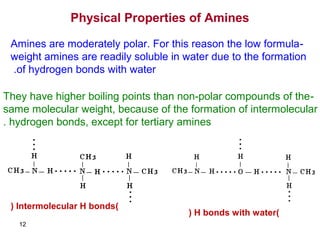
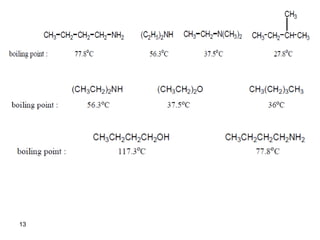
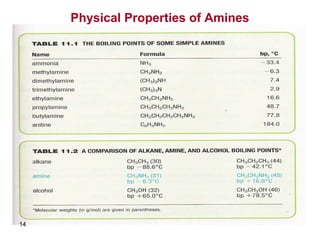
- 12. 12 Physical Properties of Amines -Amines are moderately polar. For this reason the low formula weight amines are readily soluble in water due to the formation of hydrogen bonds with water. -They have higher boiling points than non-polar compounds of the same molecular weight, because of the formation of intermolecular hydrogen bonds, except for tertiary amines. )Intermolecular H bonds( )H bonds with water(
- 13. 13
- 14. Physical Properties of Amines 14
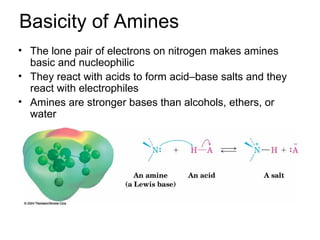
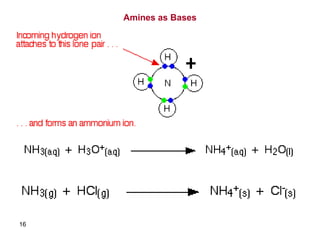
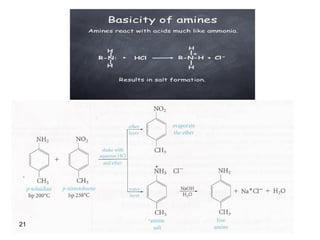
- 15. Basicity of Amines • The lone pair of electrons on nitrogen makes amines basic and nucleophilic • They react with acids to form acid–base salts and they react with electrophiles • Amines are stronger bases than alcohols, ethers, or water
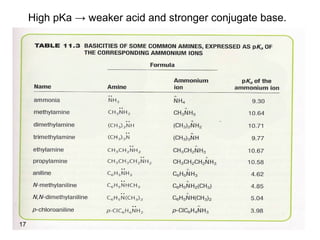
- 17. 17 High pKa → weaker acid and stronger conjugate base.
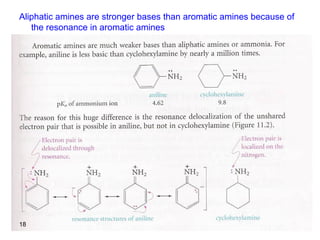
- 18. Aliphatic amines are stronger bases than aromatic amines because of the resonance in aromatic amines 18
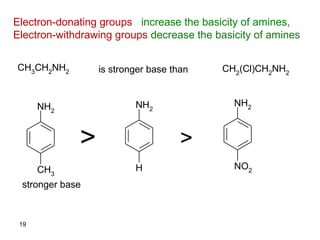
- 19. Electron-donating groups increase the basicity of amines, Electron-withdrawing groups decrease the basicity of amines CH3 CH2 NH2 CH2 (Cl)CH2 NH2 NH2 CH3 NH2 H NH2 NO2 >> is stronger base than stronger base 19
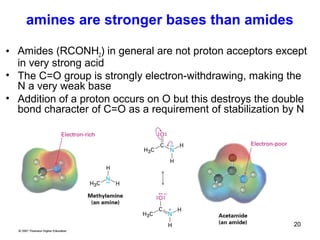
- 20. 20 amines are stronger bases than amides • Amides (RCONH2) in general are not proton acceptors except in very strong acid • The C=O group is strongly electron-withdrawing, making the N a very weak base • Addition of a proton occurs on O but this destroys the double bond character of C=O as a requirement of stabilization by N
- 21. 21
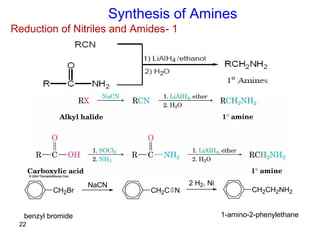
- 22. 22 1-Reduction of Nitriles and Amides Synthesis of Amines CH2Br NaCN CH2C N 2 H2, Ni CH2CH2NH2 benzyl bromide 1-amino-2-phenylethane
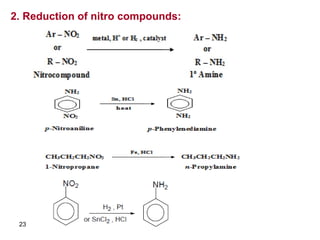
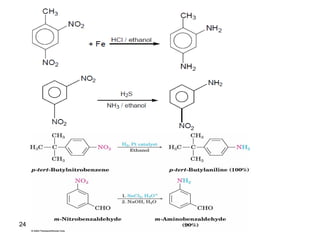
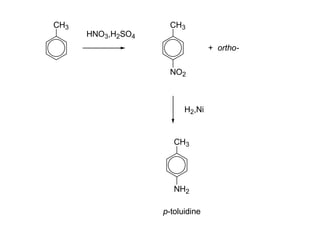
- 23. 23 2. Reduction of nitro compounds:
- 24. 24
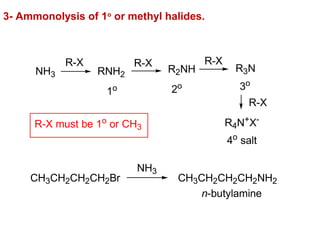
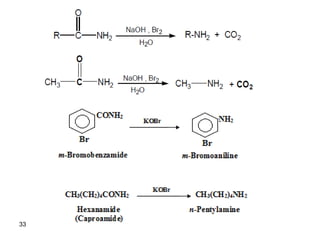
- 26. 3- Ammonolysis of 1o or methyl halides. R-X NH3 RNH2 R-X R2NH R-X R3N R-X R4N+X- 1o 2o 3o 4o salt R-X must be 1o or CH3 CH3CH2CH2CH2Br NH3 CH3CH2CH2CH2NH2 n-butylamine
- 27. 27
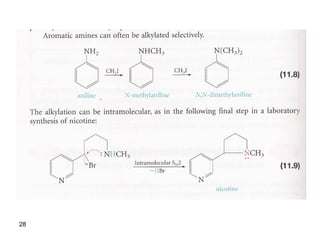
- 28. 28
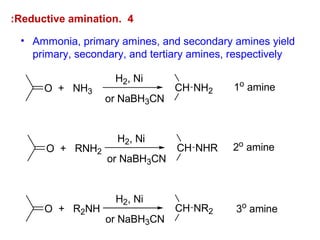
- 29. 4.Reductive amination: O H2, Ni or NaBH3CN CH NH2+ NH3 O H2, Ni or NaBH3CN CH NHR+ RNH2 O H2, Ni or NaBH3CN CH NR2+ R2NH 1o amine 3o amine 2o amine • Ammonia, primary amines, and secondary amines yield primary, secondary, and tertiary amines, respectively
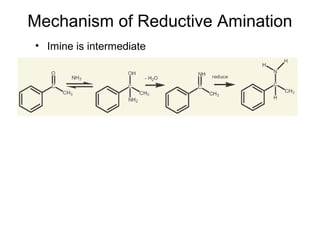
- 30. Mechanism of Reductive Amination • Imine is intermediate
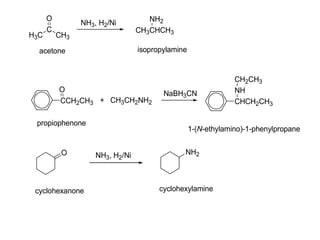
- 31. H3C C O CH3 acetone NH3, H2/Ni CH3CHCH3 NH2 isopropylamine CCH2CH3 O propiophenone + CH3CH2NH2 NaBH3CN CHCH2CH3 NH CH2CH3 1-(N-ethylamino)-1-phenylpropane O cyclohexanone NH3, H2/Ni NH2 cyclohexylamine
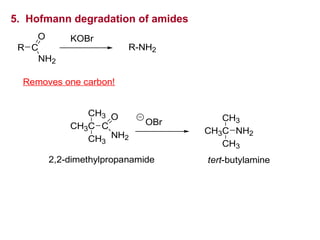
- 32. 5. Hofmann degradation of amides R C NH2 O KOBr R-NH2 Removes one carbon! 2,2-dimethylpropanamide OBr CH3C CH3 CH3 NH2 tert-butylamine CH3C CH3 CH3 C O NH2
- 33. 33
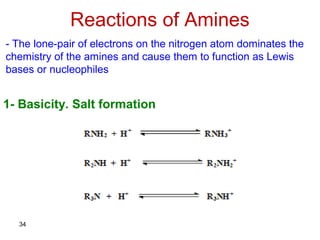
- 34. 34 Reactions of Amines - The lone-pair of electrons on the nitrogen atom dominates the chemistry of the amines and cause them to function as Lewis bases or nucleophiles 1- Basicity. Salt formation
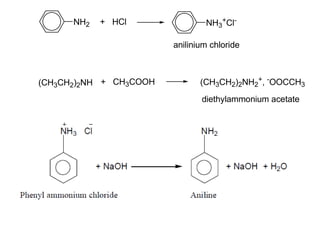
- 35. NH2 + HCl NH3 +Cl- (CH3CH2)2NH + CH3COOH (CH3CH2)2NH2 + , - OOCCH3 anilinium chloride diethylammonium acetate
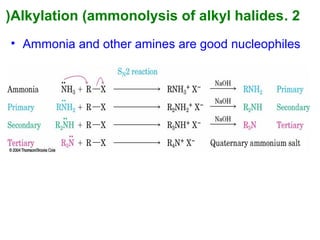
- 36. 2.Alkylation (ammonolysis of alkyl halides( • Ammonia and other amines are good nucleophiles
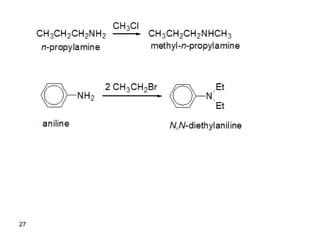
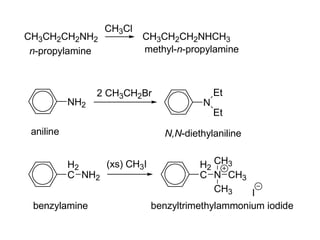
- 37. CH3CH2CH2NH2 CH3Cl CH3CH2CH2NHCH3 n-propylamine methyl-n-propylamine NH2 2 CH3CH2Br N Et Et aniline N,N-diethylaniline H2 C NH2 benzylamine (xs) CH3I H2 C N CH3 CH3 CH3 I benzyltrimethylammonium iodide
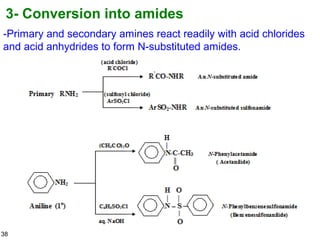
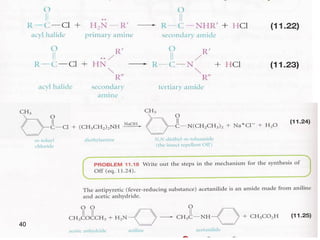
- 38. 38 3- Conversion into amides -Primary and secondary amines react readily with acid chlorides and acid anhydrides to form N-substituted amides.
- 39. 39
- 40. 40
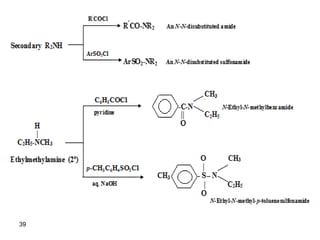
- 41. 41 -Tertiary amines do not possess a hydrogen atom bonded to nitrogen and do not form amides with acid chlorides and acid anhydrides. Hinsberg Test: unknown amine + benzenesulfonyl chloride, KOH (aq( - Reacts to produce a clear solution and then gives a ppt upon acidification primary amine. - Reacts to produce a ppt secondary amine. - Doesn’t react tertiary amine.
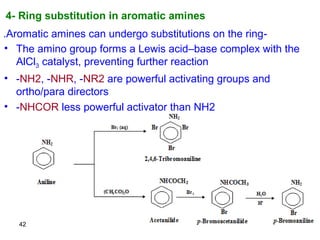
- 42. 4- Ring substitution in aromatic amines 42 -Aromatic amines can undergo substitutions on the ring. • The amino group forms a Lewis acid–base complex with the AlCl3 catalyst, preventing further reaction • -NH2, -NHR, -NR2 are powerful activating groups and ortho/para directors • -NHCOR less powerful activator than NH2
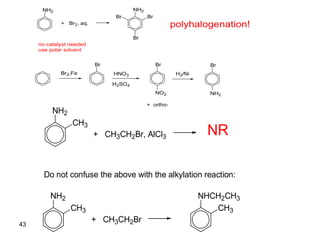
- 43. 43 NH2 + Br2, aq. NH2 Br Br Br no catalyst needed use polar solvent Br2,Fe Br HNO3 H2SO4 Br NO2 + ortho- H2/Ni Br NH2 polyhalogenation! NH2 CH3 + CH3CH2Br, AlCl3 NR Do not confuse the above with the alkylation reaction: NH2 CH3 + CH3CH2Br NHCH2CH3 CH3
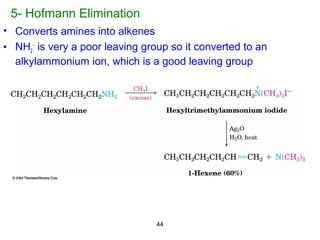
- 44. 44 5- Hofmann Elimination • Converts amines into alkenes • NH2 − is very a poor leaving group so it converted to an alkylammonium ion, which is a good leaving group
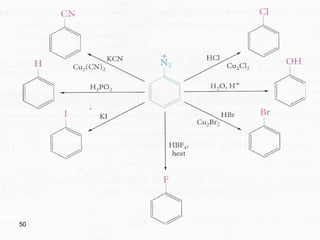
- 45. 45 6-Diazonium salts i- Reactions of Diazonium Salts 1- Replacement of nitrogen -Replacement of the diazonium group is the best general way of introducing F, Cl, Br, I, CN, OH, and H into an aromatic ring.
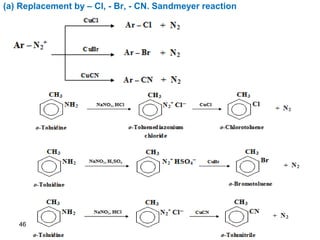
- 46. 46 (a) Replacement by – Cl, - Br, - CN. Sandmeyer reaction
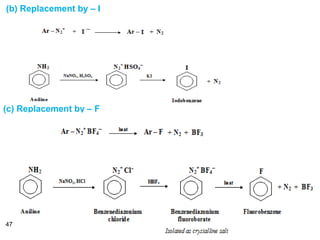
- 47. 47 (b) Replacement by – I (c) Replacement by – F
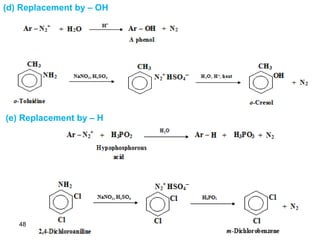
- 48. 48 (d) Replacement by – OH (e) Replacement by – H
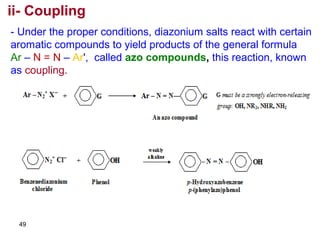
- 49. 49 ii- Coupling - Under the proper conditions, diazonium salts react with certain aromatic compounds to yield products of the general formula Ar – N = N – Ar', called azo compounds, this reaction, known as coupling.
- 50. 50
- 51. of the following compounds Draw structures 51 a( Triethyl amine b( 1,5-Pentanedi amine c( N-Isopropyl-N-methyl cyclohexyl amine d( N-Methyl aniline