Chapter 9-amines
- 1. ORGANIC CHEMISTRY CHM 207 CHAPTER 9:CHAPTER 9: AMINESAMINES NOR AKMALAZURA JANI
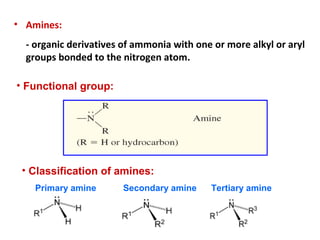
- 2. • Amines: - organic derivatives of ammonia with one or more alkyl or aryl groups bonded to the nitrogen atom. • Functional group: • Classification of amines: Primary amine Secondary amine Tertiary amine
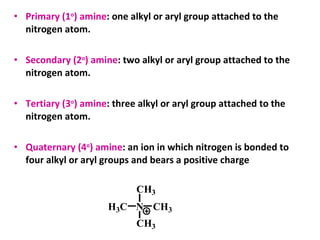
- 3. • Primary (1o ) amine: one alkyl or aryl group attached to the nitrogen atom. • Secondary (2o ) amine: two alkyl or aryl group attached to the nitrogen atom. • Tertiary (3o ) amine: three alkyl or aryl group attached to the nitrogen atom. • Quaternary (4o ) amine: an ion in which nitrogen is bonded to four alkyl or aryl groups and bears a positive charge NH3C CH3 CH3 CH3
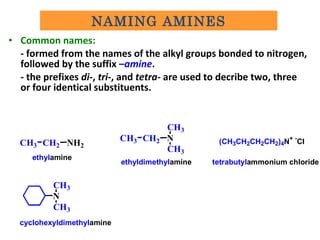
- 4. • Common names: - formed from the names of the alkyl groups bonded to nitrogen, followed by the suffix –amine. - the prefixes di-, tri-, and tetra- are used to decribe two, three or four identical substituents. CH3 CH2 NH2 ethylamine CH3 CH2 N CH3 CH3 ethyldimethylamine (CH3CH2CH2CH2)4N+ - CI tetrabutylammonium chloride N CH3 CH3 cyclohexyldimethylamine NAMING AMINES
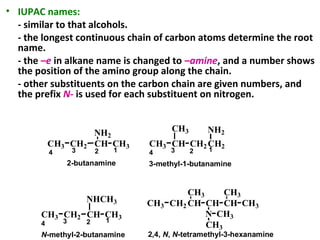
- 5. • IUPAC names: - similar to that alcohols. - the longest continuous chain of carbon atoms determine the root name. - the –e in alkane name is changed to –amine, and a number shows the position of the amino group along the chain. - other substituents on the carbon chain are given numbers, and the prefix N- is used for each substituent on nitrogen. CH3 CH2 CH CH3 NH2 CH3 CH CH3 CH2 CH2 NH2 CH3 CH2 CH CH3 NHCH3 CH2CH3 CH CH CH3 N CH CH3 CH3 CH3 CH3 2-butanamine 1234 1234 3-methyl-1-butanamine 1234 N-methyl-2-butanamine 2,4, N, N-tetramethyl-3-hexanamine
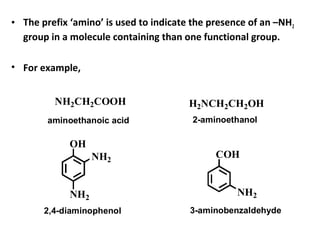
- 6. • The prefix ‘amino’ is used to indicate the presence of an –NH2 group in a molecule containing than one functional group. • For example, NH2CH2COOH OH NH2 NH2 H2NCH2CH2OH COH NH2 aminoethanoic acid 2-aminoethanol 2,4-diaminophenol 3-aminobenzaldehyde
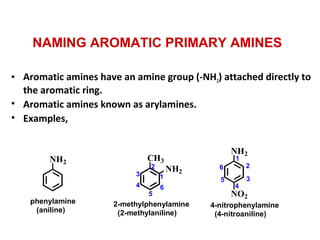
- 7. • Aromatic amines have an amine group (-NH2) attached directly to the aromatic ring. • Aromatic amines known as arylamines. • Examples, NH2 CH3 NH2 NH2 NO2 phenylamine (aniline) 2-methylphenylamine (2-methylaniline) 4-nitrophenylamine (4-nitroaniline) 1 2 3 4 5 6 1 2 3 4 5 6 NAMING AROMATIC PRIMARY AMINES
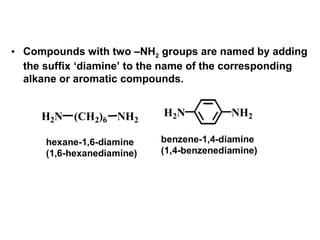
- 8. H2N (CH2)6 NH2 NH2H2N hexane-1,6-diamine (1,6-hexanediamine) benzene-1,4-diamine (1,4-benzenediamine) • Compounds with two –NH2 groups are named by adding the suffix ‘diamine’ to the name of the corresponding alkane or aromatic compounds.
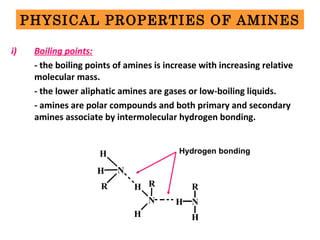
- 9. PHYSICAL PROPERTIES OF AMINES i) Boiling points: - the boiling points of amines is increase with increasing relative molecular mass. - the lower aliphatic amines are gases or low-boiling liquids. - amines are polar compounds and both primary and secondary amines associate by intermolecular hydrogen bonding. H N H R N H H R NH R H Hydrogen bonding
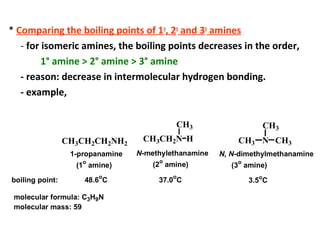
- 10. * Comparing the boiling points of 1o , 2o and 3o amines - for isomeric amines, the boiling points decreases in the order, 1° amine > 2° amine > 3° amine - reason: decrease in intermolecular hydrogen bonding. - example, CH3CH2CH2NH2 CH3CH2N CH3 H CH3 N CH3 CH3 1-propanamine (1o amine) N-methylethanamine (2 o amine) N, N-dimethylmethanamine (3o amine) boiling point: 48.6 o C 37.0 o C 3.5o C molecular formula: C3H9N molecular mass: 59
- 11. * Comparing the boiling points of amines with other organic compounds - the boiling points of aliphatic amines are higher than those of alkanes or haloalkanes of similar relative molecular mass due to intermolecular hydrogen bonding. - the N-H bond is more polar than the C-H bond but less polar than O-H bond. Hydrogen bonding in amines are weaker than that of alcohols or carboxylic acids. Boiling points of amines are lower than those corresponding alcohols or carboxylic acids. Comparison of boiling points of some organic compounds with similarComparison of boiling points of some organic compounds with similar molecular weightmolecular weight alkane < ether < alkyl halide < amine < ketone, aldehyde < alcohol < acid
- 12. ii) Solubilities of 1o , 2o and 3o amines: - all three classes of aliphatic amines are capable of forming hydrogen bonds with water molecules. - the lower amines (with chain length up to four carbon atoms per molecule) are very soluble in water because they can form hydrogen bonds with water molecules. - the solubilities of amines is decrease with increasing number of carbon atoms in the chain. - amines are soluble in organic solvents.
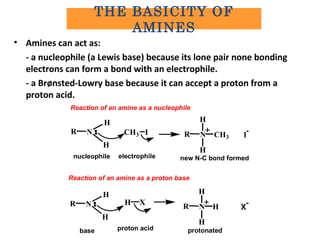
- 13. • Amines can act as: - a nucleophile (a Lewis base) because its lone pair none bonding electrons can form a bond with an electrophile. - a Brønsted-Lowry base because it can accept a proton from a proton acid. NR H H ICH3 NR H H CH3 I - nucleophile electrophile new N-C bond formed Reaction of an amine as a proton base NR H H XH NR H H H X - base proton acid protonated Reaction of an amine as a nucleophile THE BASICITY OF AMINES
- 14. • Amines are fairly strong base and their aqueous solutions are basic. • An amine can abstract a proton from water, giving an ammonium ion and a hydroxide ion. • The equilibrium constant for this reaction is called base-dissociation constant, symbolized by Kb. NR H H OH NR H H H OH - H Kb Kb = [RNH3 + ] [- OH] [RNH2] pKb = - log 10 Kb Stronger base have smaller values of pKb
- 15. • The basicity of the amines depends on the ability of the lone pair none bonding electrons at nitrogen atom to form bond with an acid. • The more easier the lone pair electrons formed bond with the acid, will make the amines a stronger base. • Factors that effect the basicity of the amines: i) substitution by alkyl groups - the presence of alkyl groups (electron-donating group) such as (CH3-) and (CH3CH2-) will make the amine become more basic. - for example, methylamine is more basic than ammonia. ii) substitution by electron-withdrawing groups - the presence of electron-withdrawing groups or atom will decrease the basicity. - for example, nitroaniline is less basic than aniline
- 16. Basicity of aromatic amines * Aromatic amines is less basic than aliphatic amines and ammonia. * Reason: - the lone pair of electrons on the nitrogen atom is delocalised into the benzene ring. - As a result, the lone pair of electrons is less available for donation to an acid. - The reaction is shifted toward the left and makes aniline a weaker base than ammonia or aliphatic amines.
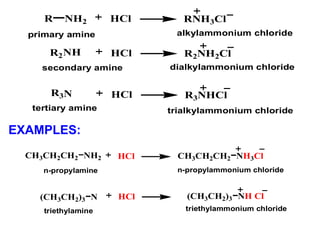
- 17. • Salt formation • Reaction with nitrous acid • Amide formation • Ring halogenation of phenylamine REACTIONS OF AMINES
- 18. • Reaction of amines and acid will give amine salt. • Amine salt: - composed of two types of ions: i) the protonated amine cation (an ammonium ion) ii) anion derived from the acid • Amine salts are ionic, have higher melting points, nonvolatile solids, more soluble in water than the parent amines and slightly soluble in nonpolar organic solvents. Salt formation
- 19. n-propylamine n-propylammonium chloride NH2CH3CH2CH2 HCl NH3ClCH3CH2CH2 N(CH3CH2)3 HCl triethylamine triethylammonium chloride NH Cl(CH3CH2)3 R NH2 HCl R2NH HCl R3N HCl R3NHCl RNH3Cl R2NH2Cl primary amine alkylammonium chloride secondary amine dialkylammonium chloride tertiary amine trialkylammonium chloride EXAMPLES:
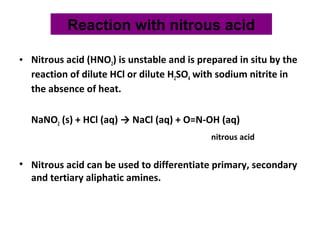
- 20. • Nitrous acid (HNO2) is unstable and is prepared in situ by the reaction of dilute HCl or dilute H2SO4 with sodium nitrite in the absence of heat. NaNO2 (s) + HCl (aq) → NaCl (aq) + O=N-OH (aq) nitrous acid • Nitrous acid can be used to differentiate primary, secondary and tertiary aliphatic amines. Reaction with nitrous acid
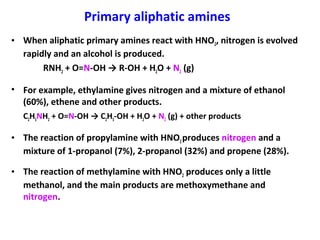
- 21. Primary aliphatic amines • When aliphatic primary amines react with HNO2, nitrogen is evolved rapidly and an alcohol is produced. RNH2 + O=N-OH → R-OH + H2O + N2 (g) • For example, ethylamine gives nitrogen and a mixture of ethanol (60%), ethene and other products. C2H5NH2 + O=N-OH → C2H5-OH + H2O + N2 (g) + other products • The reaction of propylamine with HNO2produces nitrogen and a mixture of 1-propanol (7%), 2-propanol (32%) and propene (28%). • The reaction of methylamine with HNO2 produces only a little methanol, and the main products are methoxymethane and nitrogen.
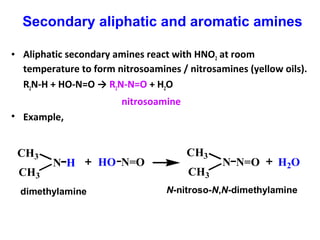
- 22. • Aliphatic secondary amines react with HNO2 at room temperature to form nitrosoamines / nitrosamines (yellow oils). R2N-H + HO-N=O → R2N-N=O + H2O nitrosoamine • Example, HN CH3 CH3 N=OHO N=ON CH3 CH3 H2O dimethylamine N-nitroso-N,N-dimethylamine Secondary aliphatic and aromatic amines
- 23. • A tertiary aliphatic amines react with HNO2 will produced ammonium salts which is dissolve readily in water as a clear solution. R3N + HNO2 → [R3NH]+ NO2 - (aq) NH2 HNO2 HCl N2 + Cl - 2H2O RT RT = room temperature N2 5o C benzenediazonium chloride mixture products Tertiary aliphatic amines Primary aromatic amines • A primary aromatic amines react with cold HNO2 and dissolved in dilute HCl at 0-5o C will produced diazonium salt. When this cold salts heated at room temperature, nitrogen gas will evolved.
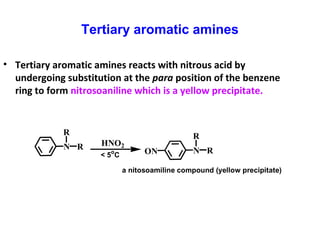
- 24. Tertiary aromatic amines N R R HNO2 N R RON a nitosoamiline compound (yellow precipitate) < 5o C • Tertiary aromatic amines reacts with nitrous acid by undergoing substitution at the para position of the benzene ring to form nitrosoaniline which is a yellow precipitate.
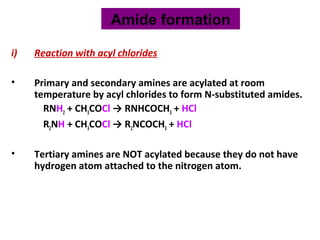
- 25. i) Reaction with acyl chlorides • Primary and secondary amines are acylated at room temperature by acyl chlorides to form N-substituted amides. RNH2 + CH3COCl → RNHCOCH3 + HCl R2NH + CH3COCl → R2NCOCH3 + HCl • Tertiary amines are NOT acylated because they do not have hydrogen atom attached to the nitrogen atom. Amide formation
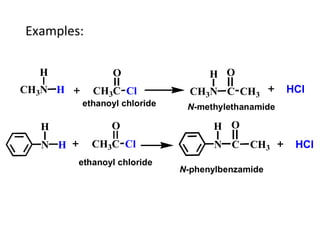
- 26. Examples: CH3N H H CH3C O Cl N H H CH3C O Cl N H C O CH3 CH3N H C CH3 O HCl HCl N-methylethanamide N-phenylbenzamide ethanoyl chloride ethanoyl chloride
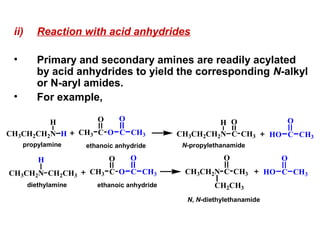
- 27. ii) Reaction with acid anhydrides • Primary and secondary amines are readily acylated by acid anhydrides to yield the corresponding N-alkyl or N-aryl amides. • For example, CH3CH2CH2N H H CCH3 O O C O CH3 CH3CH2N H CH2CH3 CCH3 O O C O CH3 CH3CH2CH2N H C O CH3 CH3CH2N C CH2CH3 CH3 O HO C O CH3 HO C O CH3 N-propylethanamideethanoic anhydride ethanoic anhydride N, N-diethylethanamide propylamine diethylamine
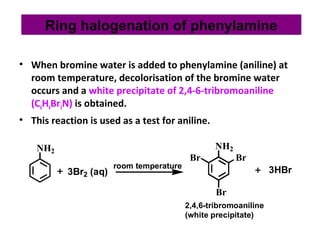
- 28. • When bromine water is added to phenylamine (aniline) at room temperature, decolorisation of the bromine water occurs and a white precipitate of 2,4-6-tribromoaniline (C6H4Br3N) is obtained. • This reaction is used as a test for aniline. NH2 3Br2 (aq) NH2 Br Br Br 3HBrroom temperature 2,4,6-tribromoaniline (white precipitate) Ring halogenation of phenylamine
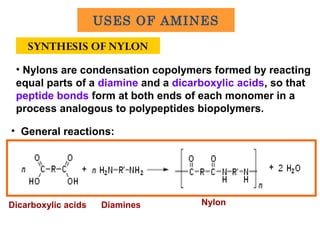
- 29. SYNTHESIS OF NYLON • General reactions: • Nylons are condensation copolymers formed by reacting equal parts of a diamine and a dicarboxylic acids, so that peptide bonds form at both ends of each monomer in a process analogous to polypeptides biopolymers. DiaminesDicarboxylic acids Nylon USES OF AMINES
- 30. Basic concepts of nylon production • The first approach: - combining molecules with an acid (COOH) group on each end are reacted with two chemicals that contain amine (NH2) groups on each end. - Form nylon 6,6, made of hexamethylene diamine with six carbon atoms and acidipic acid, as well as six carbon atoms. • The second approach: - a compound has an acid at one end and an amine at the other and is polymerized to form a chain with repeating units of (-NH-[CH2]n-CO-)x. - Form nylon 6, made from a single six-carbon substance called caprolactam.
- 31. • Primary aromatic amines are used as a starting material for the manufacture of azo dyes. • Azo compounds: - compounds bearing the functional group R-N=N-R', in which R and R' can be either aryl or alkyl. - N=N group is called an azo group - HNNH is called diimide • Aryl azo compounds have vivid colors, especially reds, oranges, and yellows SYNTHESIS OF DYE Yellow azo dye
- 32. • Amines react with nitric(III) acid to form diazonium salt, which can undergo coupling reaction to form azo compound. • Azo-compounds are highly coloured, they are widely used in dyeing industries, such as: i) Methyl orange ii) Direct brown 138 iii)Sunset yellow FCF iv)Ponceau
- 33. • Methyl orange - used as acid-base indicators due to the different colors of their acid and salt forms • Artist’s paints – clays, yellow to red range • Dye in food and textiles Uses and important of azo dye
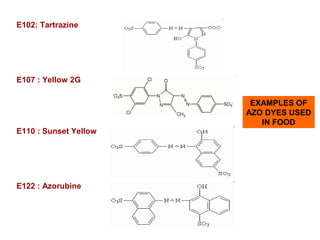
- 34. E102: Tartrazine E107 : Yellow 2G E110 : Sunset Yellow E122 : Azorubine EXAMPLES OF AZO DYES USED IN FOOD
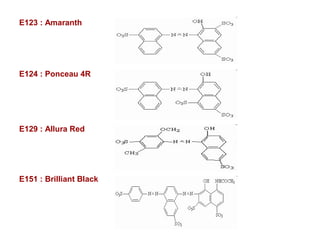
- 35. E123 : Amaranth E124 : Ponceau 4R E129 : Allura Red E151 : Brilliant Black



![• Amines are fairly strong base and their aqueous solutions are basic.
• An amine can abstract a proton from water, giving an ammonium
ion and a hydroxide ion.
• The equilibrium constant for this reaction is called base-dissociation
constant, symbolized by Kb.
NR
H
H
OH NR
H
H
H OH
-
H
Kb
Kb = [RNH3
+
] [-
OH]
[RNH2]
pKb = - log 10 Kb
Stronger base have smaller values of pKb](https://image.slidesharecdn.com/chapter-9-amines-130907082415-/85/Chapter-9-amines-14-320.jpg)




![• A tertiary aliphatic amines react with HNO2 will produced
ammonium salts which is dissolve readily in water as a clear
solution.
R3N + HNO2 → [R3NH]+
NO2
-
(aq)
NH2 HNO2 HCl N2
+
Cl
- 2H2O
RT
RT = room temperature
N2
5o
C
benzenediazonium chloride
mixture
products
Tertiary aliphatic amines
Primary aromatic amines
• A primary aromatic amines react with cold HNO2 and
dissolved in dilute HCl at 0-5o
C will produced diazonium
salt. When this cold salts heated at room temperature,
nitrogen gas will evolved.](https://image.slidesharecdn.com/chapter-9-amines-130907082415-/85/Chapter-9-amines-23-320.jpg)
![Basic concepts of nylon production
• The first approach:
- combining molecules with an acid (COOH) group on each
end are reacted with two chemicals that contain amine (NH2)
groups on each end.
- Form nylon 6,6, made of hexamethylene diamine with six
carbon atoms and acidipic acid, as well as six carbon atoms.
• The second approach:
- a compound has an acid at one end and an amine at the
other and is polymerized to form a chain with repeating units
of (-NH-[CH2]n-CO-)x.
- Form nylon 6, made from a single six-carbon substance
called caprolactam.](https://image.slidesharecdn.com/chapter-9-amines-130907082415-/85/Chapter-9-amines-30-320.jpg)